This is the first part in a feature series aimed at exploring brine and its role in ion exchange processes. In this first portion, we will take a look at the general concepts of brine makeup, including brine concentrations and its role in exchange capacity.
Brining is one of the most important steps in the water softening process and without it, ion exchange resin would be completely ineffective for most applications. Over the last few decades, the emergence of high efficiency water systems is a result of a better understanding of the kinetics of brine solutions in combination with the emergence of demand regeneration control valves and conditioning systems. High efficiency softeners depend on a strong brine solutions comprising of high purity salt that is low in impurities. A salt is defined as a chemical compound formed by the neutralization of an acid with a base. For example, in the equation NaOH (aq) + HCl (aq) --> NaCl (aq) + H2O (l), aqueous solutions of sodium hydroxide (base) and hydrochloric acid (acid) added together will produce sodium chloride (salt) and liquid water. When dissolved in water, the majority of salts will completely dissociate into the ions of which they are comprised. Considering that there is an abundance of sodium based salts and compounds available, why can't any sodium based salt be used? Sodium bicarbonate or NaHCO3 (baking soda), sodium hydroxide or NaOH (caustic soda), and sodium sequicarbonate or Na3H(CO3)2·2H2O all contain sodium. However, they cannot be used to regenerate a water softener as they would negatively impact the water chemistry and interfere with the ion exchange properties of the resin. Sodium chloride is safe, abundant, and inexpensive making it the ideal regenerant for cation and anion exchange softeners. In cation exchange softeners, sodium ions are exchanged for a chemically equivalent weight of cations (i.e. calcium, magnesium) while anion softeners exchange chloride for a chemically equivalent weight of anions (i.e. nitrate, sulfate).
In water treatment, the two most common salts used for regenerating cation and anion water softeners are sodium chloride (NaCl) and potassium chloride (KCl). Sodium chloride is less expensive and more abundant than potassium chloride, which is why it is the preferred regenerate in almost all applications. Sodium chloride is also less likely to form salt bridging in brine tanks, and is of higher purity for most manufacturers. Salt bridging is the formation of salt solidification and cementing together of salt particles in dry storage brine tanks that causes tight binding of the salt crystals. Potassium chloride should be considered for use as a regenerate when sodium content of the treated water is a concern. Most sodium chloride manufactures produce high purity salt that is anywhere from 98-99% pure salt that is crucial for developing a a good brine solution. Salt used for water conditioners is available in a variety of forms from raw rock salt to refined solar salt. Impurities in natural rock salts like calcium, magnesium, and iron can effect brine performance and regeneration capacity in a water softener. Many natural rock salts have little processing done other than grinding while others are washed, dried, and screened for improved purity. Brine creation is only as good as the salt that goes into it. Anti-caking agents, binding agents, and the purity are all important factors to consider when choosing which salt to use.
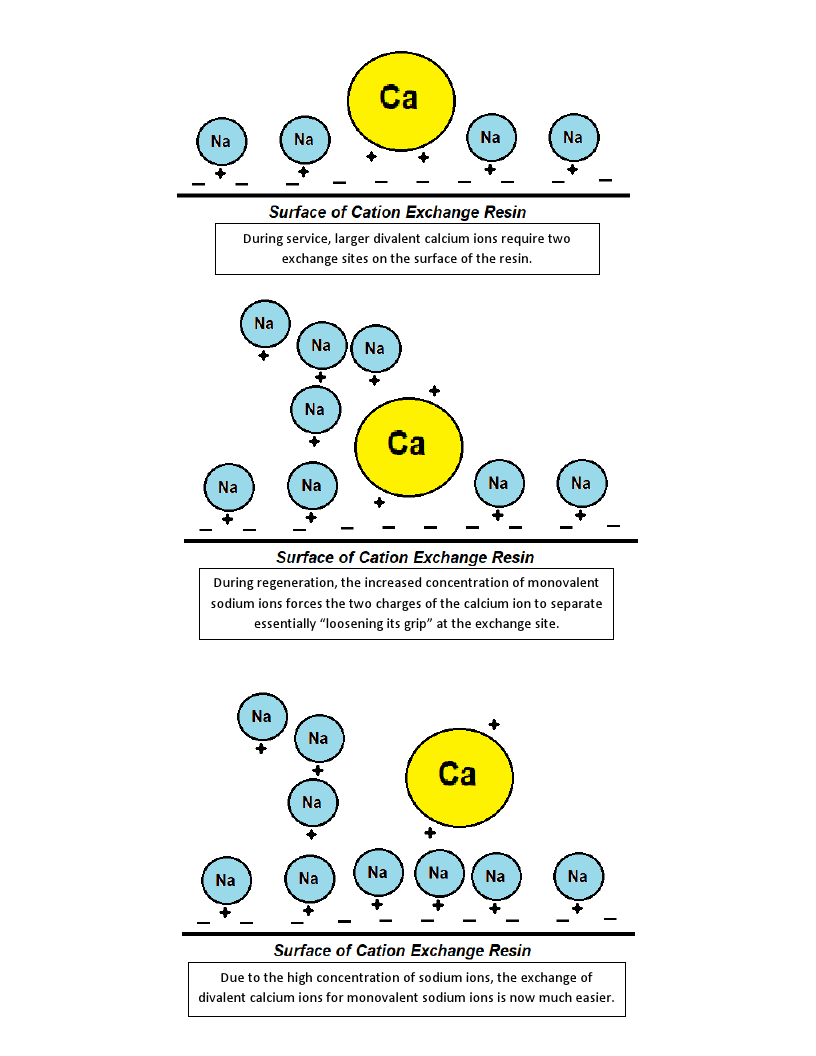
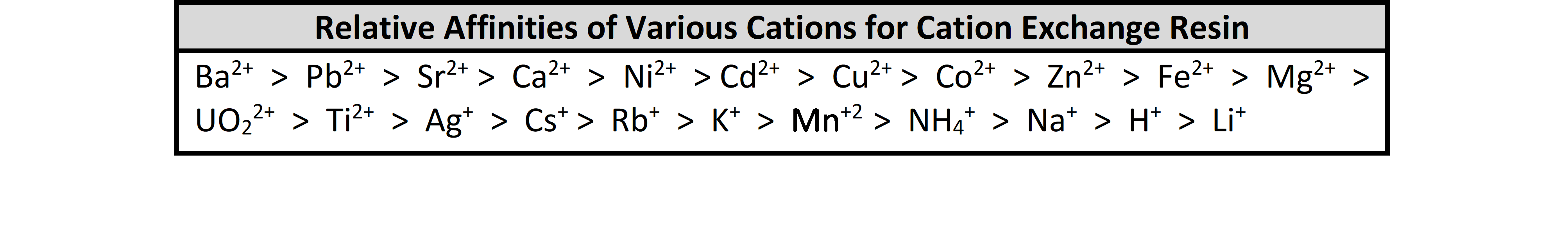
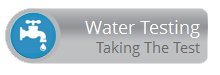
Brine solution is typically a 26% salt weight to water ratio. The 26% brine solution is then mixed with water upon entering the mineral tank and a 10-15% brine solution is what is used to regenerate the solution. Natural brine solutions exist in lower concentrations of a few thousand mg/L TDS found in some groundwater formations to sea water that has a TDS of 30,000-40,000 mg/L. Ion exchange during brine regeneration is a function of the Law of Mass Action, which states that the rate of a chemical reaction is proportional to the masses of the reacting substances. During service, the smaller, monovalent sodium ions are easily exchanged and bumped off of the resin surface for larger, multivalent hardness ions. These hardness ions require a great deal of force to be removed from the resin because of its affinity for these larger ions. During regeneration, the resin surface is overwhelmed by a high concentration of sodium ions in the brine solution that are exchanged for larger ions. Cation exchange resin has an affinity for large, multivalent ions, and only through the use of an extremely high TDS and uniform brine solution are these ions removed. Brine solutions essentially force the selectivity of the resin to shift from hardness ions to sodium ions. High capacity resins have thousands of exchange sites on their surface. When two exchange sites become available on the surface of the resin, it favors divalent hardness ions like magnesium and calcium.
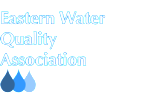
The table below illustrates this concept, as resin affinity or selectivity for sodium (Na) is lower than almost all ions that we actively try to remove from water through ion exchange.
It is important that brine solutions have a specific solution strength so that the resin can be properly cleaned during regeneration. If the brine solution strength is too low, it will not have the kinetic strength to rinse ions like calcium, magnesium, or iron off of the resin. If the brine strength is too high, excess salt will go to drain without having contributed to the regeneration process. Typically, most cation exchange softeners use anywhere from 4.5 to 15 lbs of salt per cubic foot of resin per regeneration, depending on the efficiency of the softener and the contaminants that need to be removed from the resin. Higher salt dosages and full salting are required when high levels of iron or manganese are present in the raw water. Large, multivalent ions like iron (Fe) or manganese (Mn) are difficult to remove from the resin surface with brine alone, and can cause fouling of the resin over time. Typically, a diluted strong acid solution like hydrochloric acid (HCl) or phosphoric acid (H3PO4) is added to brine solutions to remove large irons from the resin. Acids redissolve the iron or manganese back into solution so they are more easily removed from the mineral tank during regeneration.